Leider ist diese Seite derzeit nicht in deutscher Sprache verfügbar.

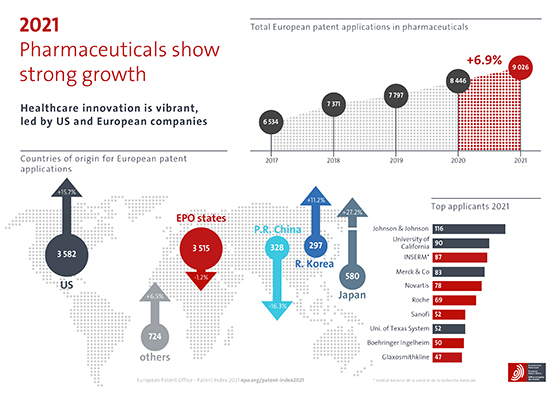
In 2021, patent applications at the European Patent Office (EPO) in the field of pharmaceuticals grew by 6.9%. The US saw a significant surge in filings and remained the top country of origin with a share of 40%. US applicants also lead the table of top applicants.
EPO member states accounted for 39% of pharmaceutical patent filings and Germany remained the leading country of origin in Europe, with an overall share of 7%. Switzerland and the previous year's growth champion France were not far behind, on 5% each. The slight decline in the absolute number of patent applications from EPO member states compared with 2020 (-1.2%) was due not least to the dip of -4.0% seen in Germany and of -18.4% in France, reversing gains made by French companies the previous year as the public research organisation INSERM dropped from first place to third among the field's top applicants.
Growth champion among the top EPO member states of 2021 was the United Kingdom (+11.9%), with a 4% share of overall filings in the field and GlaxoSmithKline entering the top ten applicants. Worldwide, Japan saw massive growth (+27.2%), albeit from a lower starting point.
The current pandemic has focussed public debate on how quickly safe vaccines can be made available, and scientific debate on the specific technologies and forms of collaboration used to develop those vaccines. Yet the complexity of the innovation ecosystems on which the success of vaccines now depends does not always come to the fore. At first glance, the top applicants ranking below would seem to reflect the bigger picture on both sides of the Atlantic, with leading pharmaceutical companies listed alongside two US universities and a French public research organisation.
Click to enlarge image
Yet the combined number of patent applications from these top
ten applicants amounts to just 8% of the grand total for the field. That is
because the field's innovation ecosystems rely heavily on numerous small and
medium enterprises (SMEs) as well as universities and public research
organisations (PROs), from which many patent-active start-ups are spun off.
A recent study published by the World Intellectual Property Organization (WIPO) showed that universities and PROs have accounted for 44% of total COVID-19 vaccine filings since the beginning of the pandemic - a far greater share than the more typical 8% of all international patent applications that such institutions filed with WIPO in 2021. For many start-ups, their knowledge and patent applications are their sole assets in the early years, prior to having a product to sell.
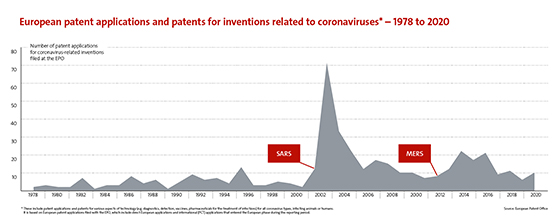
The patent system ensures that information on recent breakthroughs can be continuously shared among all actors to enable future innovation. If we dig deeper into patenting trends during the run-up to 2021, we see that research into coronavirus-related technologies in general was well advanced before the pandemic hit in 2020. This explains why vaccine developers were able to respond so fast.
Click to enlarge image
Sharp
increases in patenting activity followed both previous outbreaks of new
coronavirus infections, with patent filings peaking after Severe Acute
Respiratory Syndrome (SARS-CoV-1) hit in 2002
and, to a lesser extent, Middle East Respiratory Syndrome (MERS) in 2012.
For the purposes of tackling the current SARS-CoV-2, which causes the COVID-19 disease, it was crucial for scientists to draw on insights gained from studies of SARS and MERS viruses. There were clear indications that the spike protein would be a promising target on which to focus when developing effective vaccines because it contains the domain that recognises the receptor used by the virus to infect cells. However, the rapid and strong increase in patenting activity as a response to the COVID-19 pandemic may only become apparent in the next couple of years, as European patent applications are generally first published around 18 months after filing.
Several leading vaccine technologies have proven to be the subject of constant and steady innovation since 2000. Looking at six in detail, the only noticeable surge in patenting occurred across the board between 2014 and 2019. This coincided with outbreaks of Ebola in West Africa and of Zika in the Americas. Both ensuing epidemics lasted around 18 months and were declared over by 2016.
The six leading technologies chosen for analysis here include traditional ones based on inactivated viruses (used in influenza vaccines) and live attenuated viruses (perhaps best known for use in measles, mumps and rubella (MMR) as well as polio vaccines). Then there are vaccines based on virus-like particles (VLPs), which mimic the whole virus but do not contain the viral genome and cannot replicate. In fact, having been used to develop a vaccine for human papillomavirus, VLPs represent one of the safest technologies to have been validated to date.
 From
a structural perspective, human papillomavirus (HPV) is highly unstable and
impossible to mass-produce in the laboratory. Therefore, a vaccine based on
live viral elements is considered unfeasible. Instead, Ian
Frazer and Jian Zhou† devised the formation of "virus-like
particles" (VLPs) for their progressive vaccine. The vaccine disrupts the
link between HPV - a sexually transmitted virus infecting the skin and mucosal
tissues - and cervical cancer.
From
a structural perspective, human papillomavirus (HPV) is highly unstable and
impossible to mass-produce in the laboratory. Therefore, a vaccine based on
live viral elements is considered unfeasible. Instead, Ian
Frazer and Jian Zhou† devised the formation of "virus-like
particles" (VLPs) for their progressive vaccine. The vaccine disrupts the
link between HPV - a sexually transmitted virus infecting the skin and mucosal
tissues - and cervical cancer.
Based on Saccharomyces
cerevisiae - the brewer's yeast traditionally used to make wine and beer -
these "virus lookalikes" mimic the surface structure of HPV viral
DNA. Injected into the human body, VLPs elicit the production of 30 to 80 times
more antibodies than their natural counterparts. In 2015, the Scottish-born
Australian immunologist Ian Frazer and the late Chinese cancer researcher Jian
Zhou won
the Popular Prize at the European Inventor Award.
The massive effort prompted by the pandemic resulted in the development of COVID-19 vaccines based on various technology platforms. However, it was ultimately COVID-19 vaccines based on a fourth type of technology, namely mRNA technology, that went on to become one of the most broadly used vaccines in the world to combat the pandemic. To date, only AstraZeneca's viral vector vaccine has been used in more countries. First discovered in 1961, mRNA, or messenger ribonucleic acid, essentially teaches our cells how to make a protein, or even part of a protein. However, it took decades of research before mRNA technology could be used in vaccines to instruct cells to make spike proteins, for example, so as to provoke an immune response.
Synthesised in the laboratory for the first time in the 1980s, mRNA was long considered too unstable to have any medicinal application. Towards the end of the 2000s, scientists such as Jacek Jemielity and his team at the University of Warsaw managed to overcome this challenge by designing stable mRNA molecules. The team filed for key European patents for mRNA technology in 2008 and then established a partnership with German biopharma company BioNTech for the purposes of bringing mRNA-based therapeutics to market. BioNTech went on to license mRNA technology to major pharmaceutical companies such as French multinational Sanofi and the US-based Genentech, a subsidiary of Swiss multinational Hoffmann-La Roche. This is a typical example of how international innovation ecosystems and knowledge sharing operate in the pharmaceutical sector.
A fifth technology was required to efficiently deliver the mRNA vaccine, namely lipid nanoparticle delivery. Again, this is the product of decades of innovation, shaping the entire careers of scientists such as Patrick Couvreur, who together with three of his colleagues won the European Inventor Award in the Research category in 2013 for the delivery of cancer treatments using related technology.
In short, the advent of mRNA COVID-19 vaccines represents a regulatory breakthrough for technologies that already existed in mature form and, in the wake of the pandemic, made their way into the clinic for the first time. We may even be witnessing the beginning of a new era of mRNA vaccines and other medical applications, as discussed for example during the recent EPO Tech Day session on next-generation vaccines. Meanwhile, vaccine design is being continuously optimised through the application of artificial intelligence (AI) to bioinformatics. This means using computer science to better analyse huge volumes of biological data, including genomic data from microorganisms, in order to identify sequences of potential interest.
 Rino
Rappuoli explains how his reverse vaccinology technique has revolutionised
vaccine design since the turn of the century. The technique uses data on a pathogen's
sequenced genome to speed up the discovery of new vaccines. This in silico
(computer-simulated) approach can slash the time taken for vaccine development
solely on the basis of more traditional studies, whether in vitro (test
tube) or in vivo (using animal and human subjects).
Rino
Rappuoli explains how his reverse vaccinology technique has revolutionised
vaccine design since the turn of the century. The technique uses data on a pathogen's
sequenced genome to speed up the discovery of new vaccines. This in silico
(computer-simulated) approach can slash the time taken for vaccine development
solely on the basis of more traditional studies, whether in vitro (test
tube) or in vivo (using animal and human subjects).
There is a wealth of information on related bioinformatic filings worldwide on the EPO's Fighting coronavirus platform, which provides free public access to over 300 smart searches to support the work of clinicians, scientists and engineers. The platform is updated regularly and, in addition to various aspects of informatics, covers three further key areas: vaccines and therapeutics; diagnostics and analytics; and technologies for the new normal.
There is plenty to discover here, including in subfields such as oral or nasal vaccines. These vaccines, which may contain one or more antigens, are directly delivered to the oral or nasal mucosa and induce a local and systemic immune response. Moreover, they may be self-administered without using needles. Nasal and oral delivery have already been used for the successful delivery of other medicines, as illustrated below.
 Norwegian doctor Per Gisle Djupesland received the 2021 European
Inventor Award in the Industry category for his invention of a nasal
medication delivery system that uses the patient's own exhaled breath to
administer medication up into the nose. The invention already offers relief for
millions of migraine and chronic rhinosinusitis patients. The company founded
by Djupesland in 2000, OptiNose, has started to explore the possible use of the
system to administer a nasal antiseptic to kill the COVID-19 virus and reduce
its transmission.
Norwegian doctor Per Gisle Djupesland received the 2021 European
Inventor Award in the Industry category for his invention of a nasal
medication delivery system that uses the patient's own exhaled breath to
administer medication up into the nose. The invention already offers relief for
millions of migraine and chronic rhinosinusitis patients. The company founded
by Djupesland in 2000, OptiNose, has started to explore the possible use of the
system to administer a nasal antiseptic to kill the COVID-19 virus and reduce
its transmission.
Patents provide an important incentive for the research and development of new vaccines and medicines by enabling inventors to attract investment and recoup their R&D expenses. This is especially important in areas where product development costs are high or substantial start-up investment is required, as is the case for vaccine development.
 Furthermore, patents are very often used to facilitate co-operation
among inventors, pool R&D efforts, create standards in certain technologies
and get the latest medicines out of the lab and onto the market so that they
can benefit millions, or in the case of COVID-19 vaccines, billions of people.
A recent EPO
study on the valorisation of scientific results shows that European
universities and public research organisations use European patents as the main
instrument to exploit their inventions commercially. Finally, patents can be
enforced to stop illicit copying of medicines and the health risks associated
with counterfeit versions.
Furthermore, patents are very often used to facilitate co-operation
among inventors, pool R&D efforts, create standards in certain technologies
and get the latest medicines out of the lab and onto the market so that they
can benefit millions, or in the case of COVID-19 vaccines, billions of people.
A recent EPO
study on the valorisation of scientific results shows that European
universities and public research organisations use European patents as the main
instrument to exploit their inventions commercially. Finally, patents can be
enforced to stop illicit copying of medicines and the health risks associated
with counterfeit versions.
Once a patent has expired or lapsed in a particular territory, the invention falls into the public domain and can be used by anyone without paying royalties. However, the technical information disclosed in published patent applications is always freely available and can be used to avoid known pitfalls, for example. The EPO's online public database, Espacenet, contains more than 130 million patent documents from around the world related to inventions and technological advances.
According to WIPO, 95% of the medicines on the World Health Organization's Model List of Essential Medicines are no longer protected by a patent and are in the public domain (source: WIPO study, 2016). To learn more about patents related to coronaviruses, explore our press materials.
As technology trends in vaccine design unfold, EPO experts will continue to monitor the latest advances. The successful development of vaccines requires both strong collaboration across different technical domains, as seen during the pandemic, and continuous funding. The combined efforts of specialists across multiple fields will pay off because vaccines represent a powerful preventive treatment and serve as the basis for the most cost-effective public health strategy. In this respect, the contribution of EPO experts to sharing knowledge of the latest state-of-the-art technologies is also part of the EPO's response to the increasing urgency of achieving the UN's Sustainable Development Goals, including Goal 3: to ensure healthy lives and promote well-being for all.